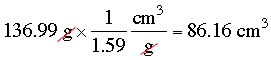| |||
| Math Central | Quandaries & Queries |
|
Question from Derrick, a student: An empty vial has a mass of 65.92 g. If the vial has a mass of 202.91 g when completely filled with liquid carbon terachloride (d = 1.59 g/cm^3), what is the volume of the vial? I have tried several solutions but none i can come up with seem to be correct. |
Hi Derrick,
Since the vial weighs 65.92 g when empty and 202.91 g when full the contents weigh 202.91 - 65.92 = 136.99 g. The density of the carbon terachloride is 1.59 g/cm3 and hence the volume is
Penny
 |
||
Math Central is supported by the University of Regina and The Pacific Institute for the Mathematical Sciences.