| |||
| Math Central | Quandaries & Queries |
|
Question from erin, a student: Hi- |
Hi Erin,
Find the specific heat is fairly simple if you know the formula:
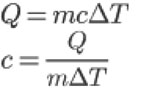 |
Where |
If you rearrange the first formula and solve for c, you can just plug in your numbers and find specific heat.
Hope this helps,
Janice
 |
||
Math Central is supported by the University of Regina and The Pacific Institute for the Mathematical Sciences.