The half-life of a radioactive isotope describes the amount of time that it takes half of the isotope in a sample to decay. In the case of radiocarbon dating, the half-life of carbon 14 is 5,730 years. This half life is a relatively small number, which means that carbon 14 dating is not particularly helpful for very recent deaths and deaths more than 50,000 years ago. After 5,730 years, the amount of carbon 14 left in the body is half of the original amount. If the amount of carbon 14 is halved every 5,730 years, it will not take very long to reach an amount that is too small to analyze. When finding the age of an organic organism we need to consider the half-life of carbon 14 as well as the rate of decay, which is –0.693.
For example, say a fossil is found that has 35% carbon 14 compared to the living sample. How old is the fossil?
We can use a formula for carbon 14 dating to find the answer.
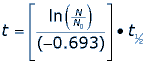
Where t1/2 is the half-life of the isotope carbon 14, t is the age of the fossil (or the date of death) and ln() is the natural logarithm function. If the fossil has 35% of its carbon 14 still, then we can substitute values into our equation.
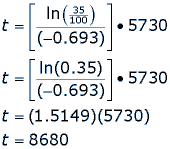
So, the fossil is 8,680 years old, meaning the living organism died 8,680 years ago.

